When energy passes, as work, as heat, or with matter, into or out from a system, its internal energy changes in accord with the law of conservation of
energy.
Equivalently, perpetual motion
machines of the
first kind are impossible.
In a natural thermodynamic
process, the
sum of the entries of the participating thermodynamic
systems increases.
Equivalently, perpetual motion
machines of the
second kind are impossible
The entropy of a system approaches a
constant value as the temperature approaches absolute zero. With the exception of glasses the entropy of a system at absolute zero is typically close
to zero, and is equal to the log of the multiplicity of the quantum ground state.
--------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
--------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
THERMODYNAMIC EQUILIBRIUM
A system is in thermodynamic equilibrium if the temperature and pressure at all pointsare same; there should be no velocity gradient ; the chemical equilibrium is also necessary.Systems under temperature and pressure equilibrium but not under chemical equilibrium aresometimes said to be in meta stable equilibrium conditions. It is only under thermodynamic equilibrium conditions that the properties of a system can be fixed.
Thus for attaining a state of thermodynamic equilibrium the following three types of equilibrium states must be achieved :
1. Thermal equilibrium.The temperature of the system does not change with time andhas same value at all points of the system.
2. Mechanical equilibrium.There are no unbalanced forces within the system or betweenthe surroundings. The pressure in the system is same at all points and does not change with respect to time.
3.Chemical equilibrium.No chemical reaction takes place in the system and the chemical composition which is same throughout the system does not vary with time.
------------------------------------------------------------------------------------------------------------------------------------------------
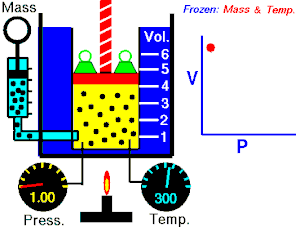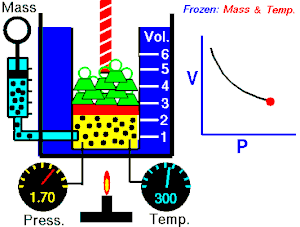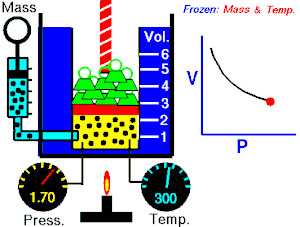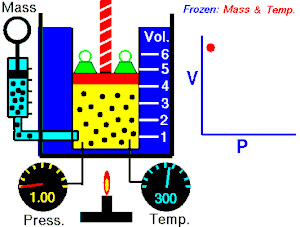
Boyle's law
The absolute pressure exerted by a given mass of an ideal/perfect gas is inversely proportional to the volume it occupies if the temperature and amount of gas remain constant within a closed system
Mathematically, Boyle's law can be stated as

-------------------------------------------------------------------------------------------------------------------------------------------------
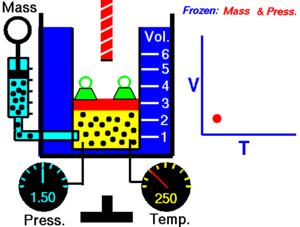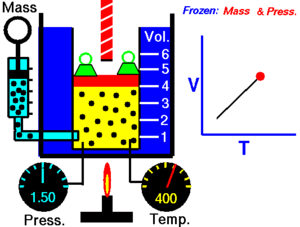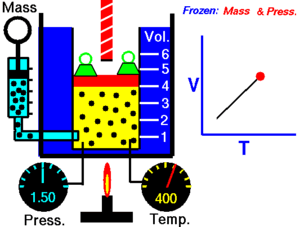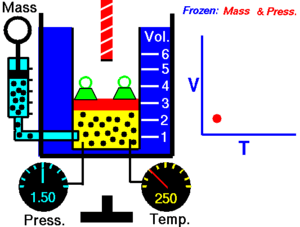
Charles's law
When the pressure on a sample of a dry gas is held constant, the Kelvin temperature and the volume will be directly related
this directly proportional relationship can be written as:
- V is the volume of the gas
- T is the temperature of the gas (measured in Kelvin).
- k is a constant.



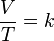
No comments:
Post a Comment